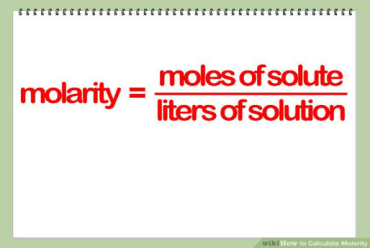
Molarity is very important in chemistry for one big reason. It is the measurement of concentration in any mixture. The molarity of any solution is a way to know the specific elements or compounds which are present in any solution. To calculate molarity, you divide the miles of a solute by the number of litters of its solutions. With molarity calculation, you can easily identify the exact amount of any element or compound in a solution.
Molarity formula
In the formula, mass is the mass of the solute represented in grams while volume is the total volume of solution represented in liters. There are many applications of molarity and calculating the dilution of a solution is one of them.
Molarity is used to explain the relationship between moles of a particular solute as well as the volume of the given solution. In order to calculate your molarity, you have to first begin with moles & volumes, moles & milliliter and mass & volume. Adding all these variables to make one formulation can be a good morality calculate to provide you with the perfect answer.
What Are The Units Of Molarity?
There is a unit of a molar concentration and it is miles per cubic decimeter. It is represented as mol/dm³ and can also be represented as M which stands for a molar. In the past, chemists represented concentration as the weight of a volume or solute but in present times, considering mole gas becomes a common way of quotation on the quantity of chemical substance, the term molarity has been used commonly instead.
It is important to note that molarity and molality are not the same terms. The latter is usually written in lower case where the former is always written in uppercase.
How to Calculate Molarity

Calculating molarity has been made a lot easier with the use of a Molarity calculator.
It is used to calculate
- The compound of a known mass to a desired concentration
- Mass of a compound necessary in preparing a solution of known concentration and volume.
- The volume of a substance needed to dissolve a compound of already known mass to the desired concentration.
In a molarity calculator, mass (m) is the amount of matter present in any substance. It has a constant value and is not affected by gravity. Molar concentration is the amount of solute which is present in a single unit of a solution. It is represented as M, molar. And formula weight is the sum of an atomic weight present in all atoms in any given empirical formula.
When it comes to making molar solutions, it might not seem like much of a hassle as long as you remember the formula accurately. However, there could be confusing times during this calculation and also during a unit conversion. An online molarity calculator has been designed to give you instant results and make it a lot more accessible and easier to calculate your molarity and acquire the necessary information.
.